The one thing to remember about battery selection is that there is no such thing as a perfect battery that works for every application. Selecting the right battery for your application is about identifying the most important battery metrics and trading these off against others. For instance, if you need a lot of power for your application, cell internal resistance needs to be minimized, and this is often done by increasing electrode surface area. But this also increases inactive components such as current collectors and conductive aid, so energy density is traded off to gain power.
While your actual design goals on the battery may be lofty, you could have to give up some things in order to gain others when it comes to actual battery performance
A lead acid battery works great in an automotive starter battery where it provides the required high rate capability. However, with its toxicity and low energy density it would be a terrible choice for a portable electronics application. So, in this 3-part blog series, we will look at how finding the right battery for your application is all about making the right tradeoffs. Part 1 discusses the important considerations when selecting the right battery for a consumer application. These include rechargeability, energy density, power density, shelf life, safety, form factor, cost and flexibility. Part 2 will look at how chemistry affects important battery metrics, and therefore battery selection for your application. In part 3 we will look at common secondary battery chemistries.
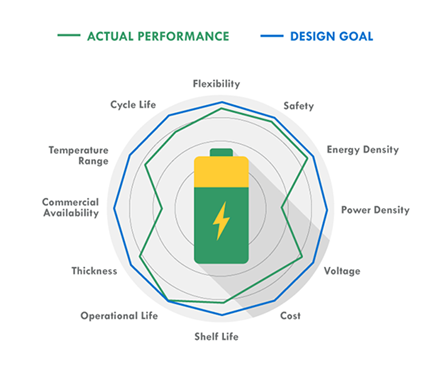
SOME IMPORTANT CONSIDERATIONS IN BATTERY SELECTION ARE:
1. Primary vs. Secondary – One of the first choices in battery selection is to decide
whether the application requires primary (single use) or secondary
(rechargeable) batteries. For the most part, this is an easy decision for the
designer. Applications with occasional intermittent use (such as a smoke alarm,
a toy or a flashlight), and disposable applications in which charging becomes
impractical warrant the use of a primary battery. Hearing aids, watches
(smartwatches being an exception), greeting cards and pacemakers are good
examples. If the battery is to be used continuously and for long stretches of
time, such as in a laptop, a cell phone or a smartwatch a rechargeable battery
is more suitable.
Primary batteries have a much lower rate of self-discharge - an attractive feature when charging is not possible or practical before first use. Secondary batteries tend to lose energy at a higher rate. This is less important in most applications because of the ability to recharge.
2. Energy vs. Power - The runtime of a battery is dictated by the battery capacity expressed in mAh or Ah and is the discharge current that a battery can provide over time.
When comparing batteries of different chemistry, it is useful to look at the energy content. To obtain the energy content of a battery, multiply the battery capacity in Ah by the voltage to obtain energy in Wh. For instance, a nickel-metal hydride battery with 1.2 V, and a lithium-ion battery with 3.2 V may have the same capacity, but the higher voltage of the lithium-ion would increase the energy.
The open circuit voltage is commonly used in energy calculations (i.e. battery voltage when not connected to a load). However, both the capacity and energy are both heavily dependent on the drain rate. Theoretical capacity is dictated only by active electrode materials (chemistry) and active mass. Yet, practical batteries achieve only a fraction of the theoretical numbers due to the presence of inactive materials and kinetic limitations, which prevent full use of active materials and buildup of discharge products on the electrodes.
Battery manufacturers often specify capacity at a given discharge rate, temperature, and cut-off voltage. The specified capacity will depend on all three factors. When comparing manufacturer capacity ratings, make sure you look at drain rates in particular. A battery that appears to have a high capacity on a spec sheet may actually perform poorly if the current drain for the application is higher. For instance, a battery rated at 2 Ah for a 20-hour discharge cannot deliver 2 A for 1 hour, but will only provide a fraction of the capacity.
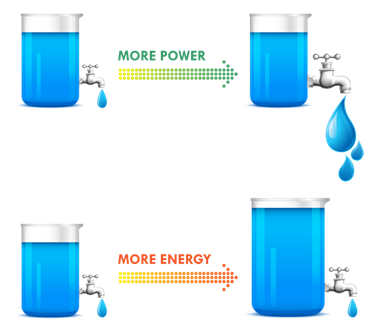
Batteries with high power provide rapid discharge capability at high drain rates such as in power tools, or automobile starter battery applications. Typically, high power batteries have low energy densities.
A good analogy for power versus energy is to think of a bucket with a spout. A larger bucket can hold more water and is akin to a battery with high energy. The opening or spout size from which the water leaves the bucket is akin to power – the higher the power, the higher the drain rate. To increase energy, you would typically increase the battery size (for a given chemistry), but to increase power you decrease internal resistance. Cell construction plays a huge part in obtaining batteries with high power density.
You should be able to compare theoretical and practical energy densities for different chemistries from battery textbooks. However, because power density is so heavily dependent on battery construction you will rarely find these values listed.
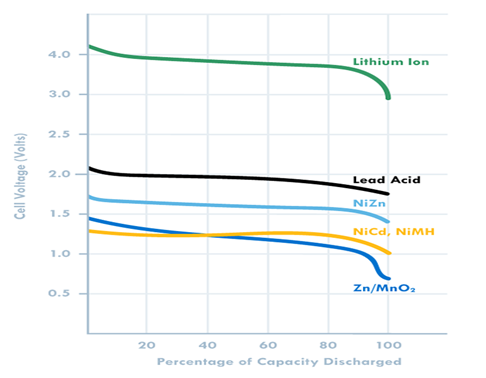
3. Voltage – Battery operating voltage is another important consideration and is dictated by the electrode materials used. A useful battery classification here is to consider aqueous or water based batteries versus lithium based chemistries. Lead acid, Zinc carbon and Nickel metal hydride all use water based electrolytes and have nominal voltages ranging from 1.2 to 2 V. Lithium based batteries, on the other hand, use organic electrolytes and have nominal voltages of 3.2 to 4 V (both primary and secondary).
Many electronic components operate at a minimum voltage of 3 V. The higher operating voltage of lithium based chemistries allows a single cell to be used rather than two or three aqueous based cells in series to make up the desired voltage.
Another thing to note is that some battery chemistries such as Zinc MnO2 have a sloping discharge curve, while others have a flat profile. This influences the cutoff voltage (Fig 3).
4. Temperature range – Battery chemistry dictates the temperature range of the application. For instance, aqueous electrolyte based Zinc-carbon cells cannot be used below 0°C. Alkaline cells also exhibit a sharp decline in capacity at these temperatures, although less than Zinc-carbon. Lithium primary batteries with an organic electrolyte can be operated up to -40°C but with a significant drop in performance.
In rechargeable applications, lithium ion batteries can be charged at maximum rate only within a narrow window of about 20° to 45°C. Beyond this temperature range, lower currents/voltages need to be used, resulting in longer charging times. At temperatures below 5° or 10°C, a trickle charge may be required in order to prevent the dreaded lithium dendritic plating problem, which increases the risk of thermal runaway (we have all heard of exploding Lithium based batteries which could happen as a result of overcharging, low or high temperature charging, or short circuiting from contaminants).
OTHER CONSIDERATIONS INCLUDE:
5. Shelf life – This refers to how long a battery will sit in a storeroom or on a shelf before it is used. Primary batteries have much longer shelf lives than secondary. However, shelf life is generally more important for primary batteries because secondary batteries have the ability to be recharged. An exception is when recharging is not practical.
6. Chemistry – Many of the properties listed above are dictated by cell chemistry. We will discuss commonly available battery chemistries in the next part of this blog series.
7. Physical size and shape – Batteries are typically available in the following size formats: button/coin cells, cylindrical cells, prismatic cells, and pouch cells (most of them in standardized formats).
8. Cost – There are times when you may need to pass up a battery with better performance characteristics because the application is very cost sensitive. This is especially true for high volume disposable applications.
9. Transportation, disposal regulations – Transportation of lithium based batteries is regulated. Disposal of certain battery chemistries is also regulated. This may be a consideration for high volume applications.
NB116 Battery 31.92Wh 3.8V Pack for Lenovo IdeaPad 100S 100S-11IBY 80R2 100S-80 R2

Details
Compatible Battery Part Number:
5B10K37675 NB116
Compatible Computer Models:
Lenovo IdeaPad 100S
Lenovo IdeaPad 100S-11IBY 80R2
Lenovo IdeaPad 100S-11IBY(80R2002HGE)
Lenovo IdeaPad 100S-11IBY(80R2002JGE)
Lenovo IdeaPad 100S-11IBY(80R2002KGE)
Lenovo IdeaPad 100S-11IBY(80R2002LGE)
Lenovo IdeaPad 100S-11IBY(80R200DHGE)
Lenovo IdeaPad 100S-80 R2
Recommend Products
1.https://www.cutebuy.com/battery/l35h-battery-2330mah-8-7wh-3-7v-pack-for-sony-xperia-zl-xperia-x-xperia-zq-c6502-c6503-zl-odin-c650x-tools.html
2.https://www.cutebuy.com/battery/bat909b-battery-1430mah-3-7dvc-pack-for-nec-nec909e-casio-g-zone-is11ca.html
3.https://www.cutebuy.com/battery/l13m4p21-l13l4p21-battery-4600mah-7-4v-pack-for-lenovo-yoga-2-11.html

