Let’s take a look at the most common secondary battery chemistries available.
1. LITHIUM ION BATTERIES
Lithium is the lightest metal in the periodic table and has a specific capacity of 3860 mAh/g compared to Zinc at 820 mAh/g (battery capacity is what gives our devices talk time or run time). Lithium also has an electrochemical reduction potential of 3.045 V against 0.76 V for Zinc (i.e a lithium based battery provides a battery voltage of 3 V or greater). The combination of these two properties results in very high energy densities for lithium based batteries.
While lithium metal based batteries could provide extremely high energy density, when these systems are charged there is the risk of dendritic growth which could penetrate the separator and create a short. We have all heard of the risks of battery explosions and thermal runaway from the resulting temperature rise associated with lithium battery shorting. This risk is alleviated in lithium ion batteries, where the lithium is in a non-metallic form, and lithium ions move back and forth between the anode and cathode. Lithium ion batteries trade-off energy density compared to lithium metal in order to be able to charge in a relatively stable and safe manner (although it is easy to forget this, considering the recent Samsung and other lithium ion battery explosions).
Owing to their excellent energy density and long cycle life (over 1000 cycles) these rechargeable systems are used in a wide variety of applications such as cell phones, laptops, plug in hybrids and electric vehicles. In the most common lithium ion battery implementation, the anode is typically a graphite sheet, the cathode a lithium cobalt oxide, and the electrolyte a lithium salt in an organic solvent.
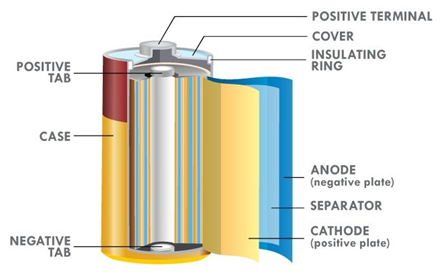
Because lithium is highly reactive towards water, it cannot be used with aqueous electrolytes unlike Zinc. Organic electrolytes are commonly used – but these pale in conductivity compared to aqueous electrolytes like potassium hydroxide, zinc chloride etc, and limit the power output of lithium batteries (in order to get power, low battery resistance and high electrolyte conductivity are required). Owing to the low conductivity of organic electrolytes, Li-based battery cell designs favor large surface area construction such as coin over button cells, and jellyroll construction over the bobbin type in order to minimize internal resistance and enhance power capability. In other words, cell construction has to be relied on to increase power output for lithium based batteries.
A high cell voltage of 3.6 Volts or greater means fewer cells are needed for high voltage applications. For example, one Lithium cell can replace three NiCad or NiMH cells which have a cell voltage of 1.2 Volts. Unlike lead acid batteries, these can be deep cycled.
Disadvantages stem from the fact that lithium is so highly reactive that special safety electronics are required. Lithium ion batteries are also subject to transportation regulations (each airline passenger is restricted to carrying 2g of metallic lithium in primary batteries or 8g of rechargeable Li-ion). Safety issues include the possibility of thermal runaway when overcharged, abused or when used outside of the operating window.
2. NICKEL METAL HYDRIDE BATTERIES
Nickel metal hydride batteries use a nickel oxyhydroxide cathode, a hydrogen absorbing alloy as anode (replaces Cd in the Ni-Cd battery) and an alkaline electrolyte. They have good high power capability, but cannot compete with lithium ion in terms of energy density. This is a 1.2 V battery system. They are resilient to both overcharge and discharge and can be operated between -30 to 75 C. While lithium ion batteries have replaced this system in many consumer applications, nickel metal hydride still finds use in hybrid electric vehicles, where it has seen more than 10 years of use. The active chemicals are also inherently safer than lithium based systems, and don’t require the use of complex battery management systems. Nickel metal hydride batteries also compete with lithium ion on cost for applications like portable tools.
3. LEAD ACID BATTERIES
Lead acid batteries have low energy densities, but are widely used in automotive starter batteries because of their ability to provide high surge currents inexpensively. No other battery system provides high power rate capability as cheaply and reliably as lead acid and this system has been in use for 140 years! No wonder this battery system finds use in applications like golf carts, forklifts, UPS vehicles, electric scooters and electric wheelchairs. Owing to their low energy density, you will never see this system used in consumer applications.
4. NICKEL CADMIUM BATTERIES
Nickel cadmium batteries have lost significant market share to Li ion and Nickel metal hydride batteries since the 1990s, due to the toxicity of cadmium. They provide a voltage of 1.2 V, and use an alkaline electrolyte. Their ability to provide high discharge rates made portable electronic applications practical when they first came to market. Advantages of this system include high cycle life, wide temperature range and their relatively high abuse tolerance. However, because of the toxicity of Cadmium, these are being phased out for many consumer applications.
BL-45A1H Battery 2300MAH/8.8Wh 3.8V/4.35V Pack for LG K10 BL-45A1H K425 K428 MS428 F670
Details
Compatible Battery Part Number:
BL-45A1H
Compatible Computer Models:
LG K10 BL-45A1H K425 K428 MS428 F670


Recommend Products
1.https://www.cutebuy.com/battery/eb-ba500abe-battery-8-74wh-2300mah-3-8v-pack-for-samsung-galaxy-a5-sm-a500-a5000-a5009.html
2.https://www.cutebuy.com/battery/bv-t5e-battery-3000mah-11-6wh-3-85v-pack-for-microsoft-lumia-950-rm-1106-rm-1104-rm-110-mcla.html
3.https://www.cutebuy.com/battery/c12p1305-battery-31wh-3-8v-pack-for-asus-transformer-pad-tf701t-k00c-tablet.html

